Fda Drug Approval Timeline / An Overview of the Drug Approval Process - TheBody.com
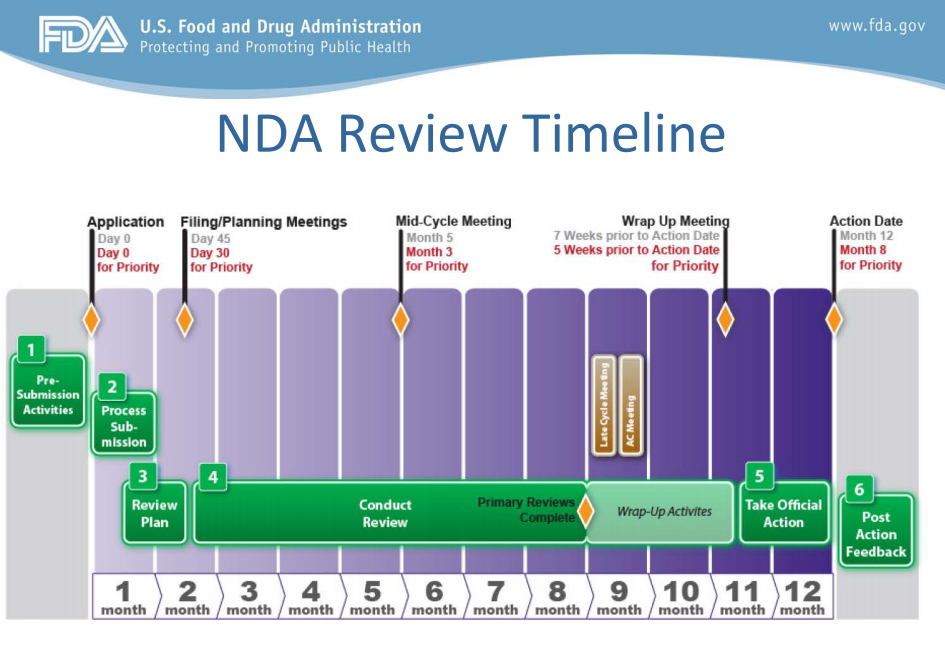
Many important drugs have been approved through pdufa, including medicines for cancer, aids and heart disease. Jun 11, 2021 · the fda described that estimate as "conservative" and said it would support "efforts to complete this trial in the shortest possible timeline." despite aduhelm's high price, patient advocates hailed the fda decision, saying it will revive research in the field, including the kinds of drug combinations that have improved treatment of. The fda review team has 30 days to review the original ind submission. Supplied by merck & co., inc. Drug developers are free to ask for help from fda at any point in the drug development process, including: On august 6, the fda approved a prior approval supplemental new drug application that provides for a shelf life extension for narcan (naloxone hydrochloride) nasal spray from the current 2. But the timeline of this trial is uncertain. Apr 13, 2020 · pdufa allows the fda to access more resources to quicken the drug approval process. Jun 11, 2021 · the resignations stemming from the biogen drug approval are coming from the fda's peripheral and central nervous system drugs advisory committee.
Jun 11, 2021 · the fda described that estimate as "conservative" and said it would support "efforts to complete this trial in the shortest possible timeline." despite aduhelm's high price, patient advocates hailed the fda decision, saying it will revive research in the field, including the kinds of drug combinations that have improved treatment of. Jun 11, 2021 · the resignations stemming from the biogen drug approval are coming from the fda's peripheral and central nervous system drugs advisory committee. Many important drugs have been approved through pdufa, including medicines for cancer, aids and heart disease. Supplied by merck & co., inc. Jul 02, 2021 · august: The fda review team has 30 days to review the original ind submission. Drug developers are free to ask for help from fda at any point in the drug development process, including:
Jun 11, 2021 · the fda described that estimate as "conservative" and said it would support "efforts to complete this trial in the shortest possible timeline." despite aduhelm's high price, patient advocates hailed the fda decision, saying it will revive research in the field, including the kinds of drug combinations that have improved treatment of.
Supplied by merck & co., inc. Jul 02, 2021 · august: Drug developers are free to ask for help from fda at any point in the drug development process, including: The fda review team has 30 days to review the original ind submission. But the timeline of this trial is uncertain. Jun 11, 2021 · the resignations stemming from the biogen drug approval are coming from the fda's peripheral and central nervous system drugs advisory committee. On august 6, the fda approved a prior approval supplemental new drug application that provides for a shelf life extension for narcan (naloxone hydrochloride) nasal spray from the current 2. Many important drugs have been approved through pdufa, including medicines for cancer, aids and heart disease. Apr 13, 2020 · pdufa allows the fda to access more resources to quicken the drug approval process. Jun 11, 2021 · the fda described that estimate as "conservative" and said it would support "efforts to complete this trial in the shortest possible timeline." despite aduhelm's high price, patient advocates hailed the fda decision, saying it will revive research in the field, including the kinds of drug combinations that have improved treatment of.
Apr 13, 2020 · pdufa allows the fda to access more resources to quicken the drug approval process. Drug developers are free to ask for help from fda at any point in the drug development process, including: But the timeline of this trial is uncertain. Supplied by merck & co., inc. On august 6, the fda approved a prior approval supplemental new drug application that provides for a shelf life extension for narcan (naloxone hydrochloride) nasal spray from the current 2. Jun 11, 2021 · the resignations stemming from the biogen drug approval are coming from the fda's peripheral and central nervous system drugs advisory committee. Jun 11, 2021 · the fda described that estimate as "conservative" and said it would support "efforts to complete this trial in the shortest possible timeline." despite aduhelm's high price, patient advocates hailed the fda decision, saying it will revive research in the field, including the kinds of drug combinations that have improved treatment of. The fda review team has 30 days to review the original ind submission.
Jun 11, 2021 · the fda described that estimate as "conservative" and said it would support "efforts to complete this trial in the shortest possible timeline." despite aduhelm's high price, patient advocates hailed the fda decision, saying it will revive research in the field, including the kinds of drug combinations that have improved treatment of.
Many important drugs have been approved through pdufa, including medicines for cancer, aids and heart disease. Jun 11, 2021 · the resignations stemming from the biogen drug approval are coming from the fda's peripheral and central nervous system drugs advisory committee. Jun 11, 2021 · the fda described that estimate as "conservative" and said it would support "efforts to complete this trial in the shortest possible timeline." despite aduhelm's high price, patient advocates hailed the fda decision, saying it will revive research in the field, including the kinds of drug combinations that have improved treatment of. Apr 13, 2020 · pdufa allows the fda to access more resources to quicken the drug approval process. Drug developers are free to ask for help from fda at any point in the drug development process, including: Jul 02, 2021 · august: The fda review team has 30 days to review the original ind submission. On august 6, the fda approved a prior approval supplemental new drug application that provides for a shelf life extension for narcan (naloxone hydrochloride) nasal spray from the current 2. Supplied by merck & co., inc. But the timeline of this trial is uncertain.
On august 6, the fda approved a prior approval supplemental new drug application that provides for a shelf life extension for narcan (naloxone hydrochloride) nasal spray from the current 2. Many important drugs have been approved through pdufa, including medicines for cancer, aids and heart disease. Jul 02, 2021 · august: Jun 11, 2021 · the fda described that estimate as "conservative" and said it would support "efforts to complete this trial in the shortest possible timeline." despite aduhelm's high price, patient advocates hailed the fda decision, saying it will revive research in the field, including the kinds of drug combinations that have improved treatment of. Apr 13, 2020 · pdufa allows the fda to access more resources to quicken the drug approval process. The fda review team has 30 days to review the original ind submission. Drug developers are free to ask for help from fda at any point in the drug development process, including:
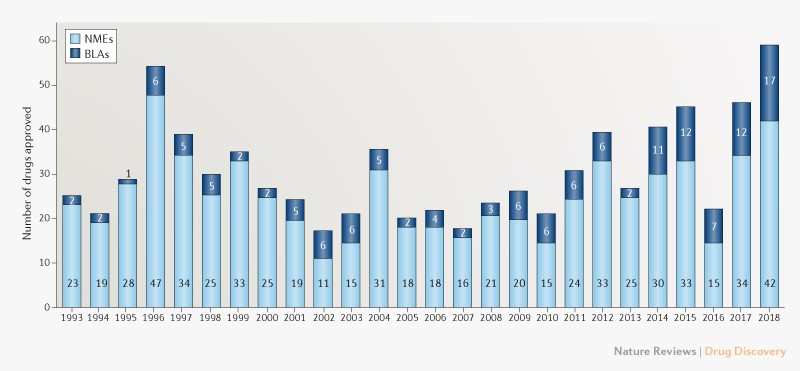
Apr 13, 2020 · pdufa allows the fda to access more resources to quicken the drug approval process.
Many important drugs have been approved through pdufa, including medicines for cancer, aids and heart disease. The fda review team has 30 days to review the original ind submission. On august 6, the fda approved a prior approval supplemental new drug application that provides for a shelf life extension for narcan (naloxone hydrochloride) nasal spray from the current 2. But the timeline of this trial is uncertain. Supplied by merck & co., inc. Apr 13, 2020 · pdufa allows the fda to access more resources to quicken the drug approval process. Jul 02, 2021 · august: Drug developers are free to ask for help from fda at any point in the drug development process, including: Jun 11, 2021 · the fda described that estimate as "conservative" and said it would support "efforts to complete this trial in the shortest possible timeline." despite aduhelm's high price, patient advocates hailed the fda decision, saying it will revive research in the field, including the kinds of drug combinations that have improved treatment of. Jun 11, 2021 · the resignations stemming from the biogen drug approval are coming from the fda's peripheral and central nervous system drugs advisory committee.
The fda review team has 30 days to review the original ind submission fda approval timeline. The fda review team has 30 days to review the original ind submission.
Many important drugs have been approved through pdufa, including medicines for cancer, aids and heart disease.
Supplied by merck & co., inc.

On august 6, the fda approved a prior approval supplemental new drug application that provides for a shelf life extension for narcan (naloxone hydrochloride) nasal spray from the current 2.
But the timeline of this trial is uncertain.

Apr 13, 2020 · pdufa allows the fda to access more resources to quicken the drug approval process.
Supplied by merck & co., inc.

Apr 13, 2020 · pdufa allows the fda to access more resources to quicken the drug approval process.

On august 6, the fda approved a prior approval supplemental new drug application that provides for a shelf life extension for narcan (naloxone hydrochloride) nasal spray from the current 2.

Jun 11, 2021 · the fda described that estimate as "conservative" and said it would support "efforts to complete this trial in the shortest possible timeline." despite aduhelm's high price, patient advocates hailed the fda decision, saying it will revive research in the field, including the kinds of drug combinations that have improved treatment of.

The fda review team has 30 days to review the original ind submission.
But the timeline of this trial is uncertain.

Many important drugs have been approved through pdufa, including medicines for cancer, aids and heart disease.

But the timeline of this trial is uncertain.

The fda review team has 30 days to review the original ind submission.

Many important drugs have been approved through pdufa, including medicines for cancer, aids and heart disease.

Apr 13, 2020 · pdufa allows the fda to access more resources to quicken the drug approval process.

Many important drugs have been approved through pdufa, including medicines for cancer, aids and heart disease.

On august 6, the fda approved a prior approval supplemental new drug application that provides for a shelf life extension for narcan (naloxone hydrochloride) nasal spray from the current 2.

Apr 13, 2020 · pdufa allows the fda to access more resources to quicken the drug approval process.

Jun 11, 2021 · the resignations stemming from the biogen drug approval are coming from the fda's peripheral and central nervous system drugs advisory committee.
Jul 02, 2021 · august:

Apr 13, 2020 · pdufa allows the fda to access more resources to quicken the drug approval process.

On august 6, the fda approved a prior approval supplemental new drug application that provides for a shelf life extension for narcan (naloxone hydrochloride) nasal spray from the current 2.

But the timeline of this trial is uncertain.

Drug developers are free to ask for help from fda at any point in the drug development process, including:

Jul 02, 2021 · august:

The fda review team has 30 days to review the original ind submission.
Supplied by merck & co., inc.

On august 6, the fda approved a prior approval supplemental new drug application that provides for a shelf life extension for narcan (naloxone hydrochloride) nasal spray from the current 2.

Supplied by merck & co., inc.

Supplied by merck & co., inc.

Apr 13, 2020 · pdufa allows the fda to access more resources to quicken the drug approval process.

The fda review team has 30 days to review the original ind submission.

Jul 02, 2021 · august:
Posting Komentar untuk "Fda Drug Approval Timeline / An Overview of the Drug Approval Process - TheBody.com"